EISAI TO PRESENT THE LATEST ALZHEIMER’S DISEASE PIPELINE AND RESEARCH, INCLUDING LECANEMAB AND ANTI-MTBR TAU ANTIBODY E2814, AT THE ALZHEIMER’S ASSOCIATION INTERNATIONAL CONFERENCE (AAIC) 2023
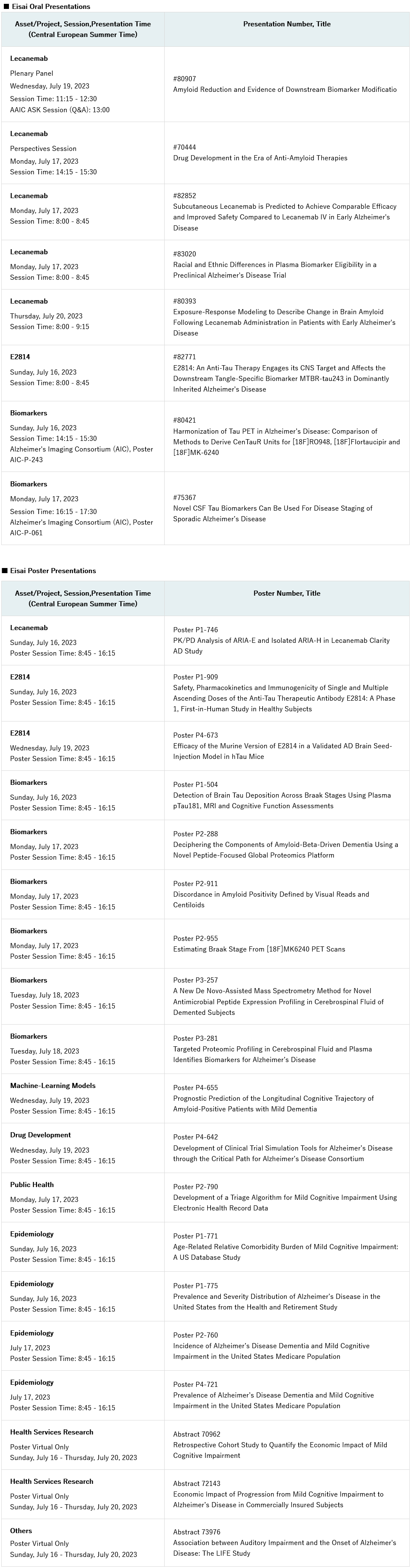
Eisai Co. Ltd (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that the company will present the latest findings on its Alzheimer’s disease (AD) pipeline and research, including Eisai’s anti-amyloid beta (Aβ) protofibril* antibody for the treatment of Alzheimer’s disease (AD), lecanemab (generic name, U.S. brand name: LEQEMBI®), and the company’s investigational anti-MTBR** tau antibody, E2814, at the Alzheimer’s Association International Conference (AAIC). The conference will be held in Amsterdam, the Netherlands and virtually from July 16 to 20, 2023. Eisai will present data and research in eight oral and 19 poster presentations at the meeting. Two of the AAIC oral presentations will be presented as posters at the Alzheimer’s Disease Imaging Consortium (AIC), which will be held at the same venue as AAIC on July 15.
“At AAIC 2023 Eisai will present the latest data on lecanemab, an anti-Aβ protofibril antibody, that recently received traditional approval in the U.S. for patients with mild cognitive impairment (MCI) due to AD and mild AD. Leqembi was studied in a broad population, which included a mix of racial and ethnic groups and patients with common comorbid conditions and concomitant medications.” Additionally, Eisai will present important new data on E2814, an anti-MTBR tau antibody, which is currently in Phase II/III clinical trials with the Dominantly Inherited Alzheimer’s Network Trials Unit at Washington University St. Louis,” said Michael Irizarry, M.D., Deputy Chief Clinical Officer and Senior Vice President of Clinical Research, Alzheimer’s Disease and Brain Health, Eisai Inc. “As part of Eisai’s commitment to transparency and our human health care (hhc) and ecosystem mission, we will continue to present and publish data and information about our AD pipeline and research.”
Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
This release discusses investigational uses of agents in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945
Libby_Holman@Eisai.com
Eisai Europe, Ltd.
(UK, Europe, Australia, New Zealand and Russia)
EMEA Communications Department
+44 (0) 786 601 1272
EMEA-comms@eisai.net
