EISAI PRESENTS RESULTS OF PHASE III CLINICAL STUDY OF LENVIMA® (LENVATINIB) IN UNRESECTABLE HEPATOCELLULAR CARCINOMA AT 11TH ILCA ANNUAL CONFERENCE
RESULTS OF SUBPOPULATION ANALYSIS OF PATIENTS WITH HEPATITIS B VIRUS COINFECTION
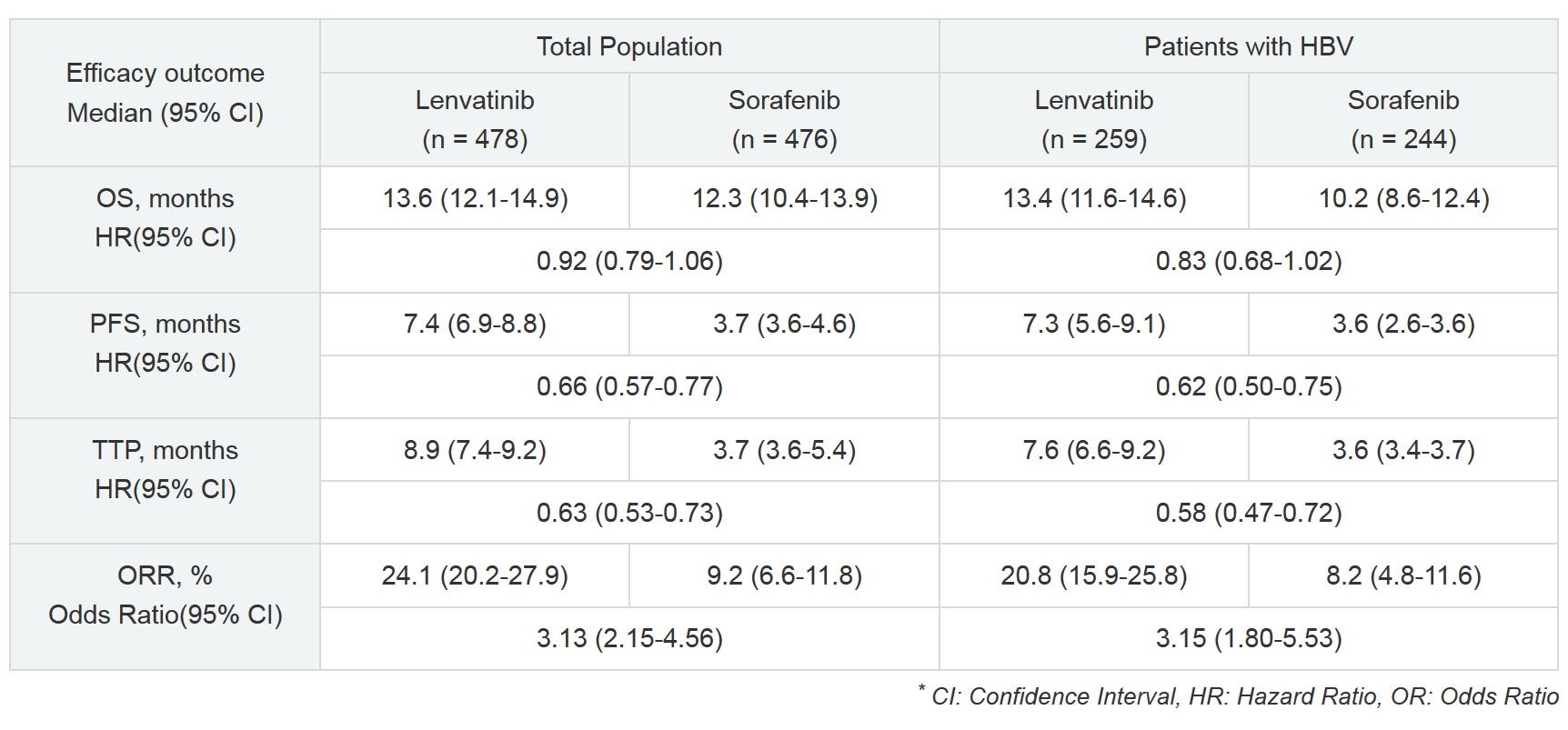
In the total population analysis of this study, the treatment effect of lenvatinib for all patients on the primary endpoint of Overall Survival (OS) was demonstrated by statistical confirmation of non-inferiority to sorafenib. Lenvatinib showed highly statistically significant and clinically meaningful improvements in the secondary endpoints of Progression Free Survival (PFS), Time To Progression (TTP), and Objective Response Rate (ORR).
The analysis results presented at ILCA indicate that in comparison to the total population, lenvatinib demonstrated a lower hazard ratio for OS, FPS, and TTP, and a higher odds ratio for ORR in the subpopulation of patients with HBV (See table below). HBV is considered to be a negative predictor of tumor response to existing drug therapies, so lenvatinib, which demonstrated a therapeutic effect in patients with HBV, is expected to be a new treatment option for patients with HCC.
Additionally, safety results were similar in patients with HBV and the total population in the lenvatinib arm. The five most common treatment-emergent adverse events in patients with HBV in the lenvatinib arm were hypertension, diarrhea, decreased weight, fatigue and decreased appetite.
Liver cancer is the second leading cause of cancer related deaths and is estimated to be responsible for 750,000 deaths per year globally. Additionally, 780,000 cases are newly diagnosed each year, about 80% of which occur in Asian regions. HCC accounts for 85% to 90% of primary liver cancer cases. Treatment options for unresectable HCC are limited and the prognosis is very poor, making this an area of high unmet medical need.
Following submissions in Japan (June 2017), the United States, and Europe (July 2017), Eisai will submit a regulatory application for lenvatinib in HCC in China within the latter half of fiscal 2017.
Eisai remains committed to generating scientific evidence aimed at maximizing the value of lenvatinib as it seeks to contribute further to addressing the diverse needs of, and increasing the benefits provided to, patients with cancer, their families, and healthcare providers.
