EISAI PRESENTS LATEST DATA ON BACE INHIBITOR ELENBECESTAT(E2609)AT 9TH CLINICAL TRIALS ON ALZHEIMER’S DISEASE
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that it has presented the latest two clinical trials (Study 202 and Study 006) data on its in-house discovered oral BACE (beta amyloid cleaving enzyme ) inhibitor elenbecestat (development code: E2609) at the 9th Clinical Trials on Alzheimer’s Disease (CTAD 2016) held from December 8 to 10 in San Diego, the United States.
Study 202 is a multicenter, randomized, double-blind, placebo-controlled parallel-group phase II clinical study to evaluate the safety of elenbecestat and the change from baseline in cerebrospinal fluid (CSF) amyloid beta Aβ(1-x)* level in patients with mild cognitive impairment (MCI) due to Alzheimer’s disease or mild to moderate dementia due to Alzheimer’s disease with confirmed accumulation of amyloid beta (Aβ) by PET (positron emission tomography) screening. Patients are administered 5, 15 or 50 mg of elenbecestat daily. The change in Aβ(1-x) level is evaluated by analyzing the concentrations of Aβ(1-x) in plasma and CSF before and after elenbecestat administration. The presentation highlighted the results from a preliminary analysis of pharmacokinetic and pharmacodynamics data of Study 202 at the CTAD 2016 (poster presentation number: P3-28).
In Study 202, the pharmacokinetic profile of elenbecestat was similar to the results obtained from Phase I studies in healthy volunteers. A correlation between plasma concentration of elenbecestat and the decrease in CSF Aβ(1-x) was observed, which overlapped with exposure-response models combining data from this study and Phase I clinical study data (figure 1).
* Aβ(1-x) refers to Aβ peptides of all lengths
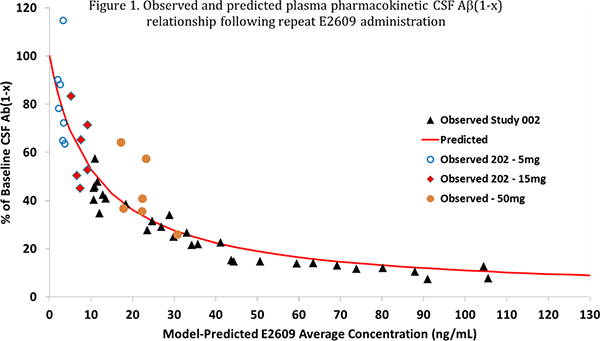
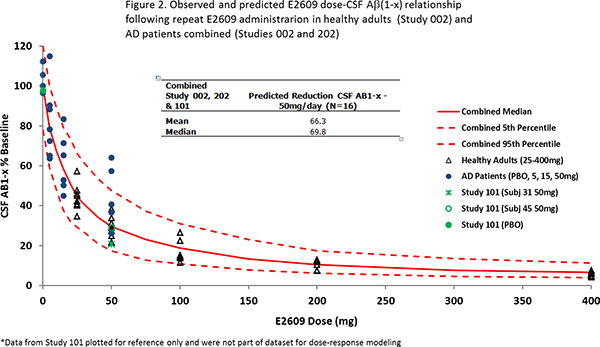
Furthermore, a dose-response model was established to explain the relationship between dosage of elenbecestat and the decrease in CSF Aβ(1-x) using the same dataset. This model was able to describe the decrease in CSF Aβ(1-x) in Study 202 well. The predicted median reduction in CSF Aβ(1-x) at 50 mg/day dose of elenbecestat was 70% (figure 2). Based upon these results, a global Phase III clinical study (MISSION AD) is currently underway to confirm efficacy and safety of elenbecestat at a dosage of 50 mg/day.
Study 006 was a bridging study (Phase I clinical study) of elenbecestat to investigate similarity of the pharmacokinetics, pharmacodynamics and safety profiles between Japanese and white healthy subjects (poster presentation number: P3-27). The study was conducted as a randomized, double-blind, placebo-controlled study. Single oral doses of 5, 50 or 200 mg/day of elenbecestat were administered in healthy adult Japanese and 50 mg/day of elenbecestat in white subjects, respectively.
From the results of the study, a dose-dependent decrease in plasma Aβ(1-x) level was observed in Japanese subjects. Pharmacokinetic and pharmacodynamics profiles were similar between Japanese and white subjects. There were no specific racial differences in safety findings.
Discovered in-house by Eisai, elenbecestat is an investigational oral BACE inhibitor currently being investigated in a Phase III clinical study for Alzheimer’s disease. By inhibiting BACE, a key enzyme in the production of Aβ peptides, elenbecestat decreases the formation of these peptides which can aggregate into toxic oligomers and protofibrils and eventually form amyloid plaques in the brain. It is believed that decreasing the formation of these plaques may potentially slow disease progression. Elenbecestat is being jointly developed by Eisai and Biogen Inc. (Headquarters: Massachusetts, United States, CEO: George A. Scangos, “Biogen”). In addition, the U.S. Food and Drug Administration has granted Fast Track designation for the development of elenbecestat.
Eisai considers dementia a therapeutic area of focus and is committed to new drug such as elenbecestat development in this field. Eisai is striving to bringing promising therapies to patients worldwide as early as possible.
